Why Does UV Light Make Things Glow?
Ultraviolet light creates some really strange and beautiful glows from all kinds of surfaces, yet not everything lights up. So, why does UV light make things glow?
Fluorescence under UV involves some interesting physics associated with parts of atoms called electrons…
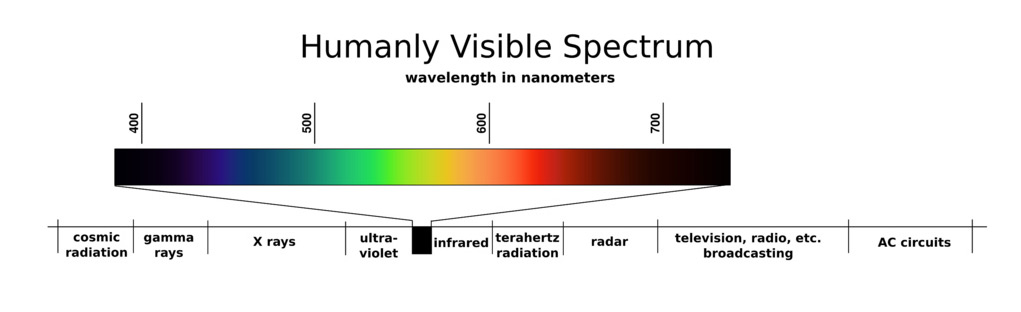
Starting with the basics, typical household ultraviolet lights, whether tube, bulb or LED, all emit light that falls into the part of the electromagnetic spectrum next to the visible (400-700nm) section called ultraviolet. You can see the UV region just left of the blacked out visible section in the diagram below:
“Black light” is what is usually emitted from non-commercial, household UV devices. Lights such as these perform a wide range of really useful functions such as locating hidden pet stains, producing super visuals at UV parties, or detecting counterfeit bank notes. Black lights are readily available because they’re quite safe, being longer in wavelength than the much more damaging shorter wavelengths of 320nm and below. Black light, so called because of its dull, low visible light emission, is a sub-section of UV light, residing in the upper/longer wavelength end of the UV range. This range of light is known as “UVA”, and falls within what is known as “near ultraviolet”. The complete range of UV light classifications (some overlap!) can be seen below:
| Name | Abbreviation | Wavelength range |
| Ultraviolet A | UVA | 400 – 315 nm |
| Ultraviolet B | UVB | 315 – 280 nm |
| Ultraviolet C | UVC | 280 – 100 nm |
| Near Ultraviolet | NUV | 400 – 300 nm |
| Middle Ultraviolet | MUV | 300 – 200 nm |
| Far Ultraviolet | FUV | 200 – 122 nm |
| Hydrogen Lyman-alpha | H Lyman-a | 122 – 121 nm |
| Extreme Ultraviolet | EUV | 121 – 10 nm |
| Vacuum Ultraviolet | VUV | 200 – 10 nm |
The glow that you see when you shine UV light over a surface comes from “phosphors”. Phosphors are substances, typically with a strong/rigid atomic structure and prevalence of delocalized (see this URL for an in-depth explanation: http://en.wikipedia.org/wiki/Delocalized_electron) electrons, that emit light when exposed to radiation. In this case, the light from phosphorescence is in the visible part of the electromagnetic spectrum. Below is an example of phosphorescence from a home mineral display:
Energy from UV light is initially absorbed by electrons in these phosphors, forcing some electrons to jump into new, farther out and much more unstable orbits around the atomic nuclei they’re part of. Whilst in these faster, more furious, energy rich orbits some energy starts to escape them, cooling and slowing down their pace. This cooling eventually forces the electrons to fall back into their original, more stable orbits. It is primarily this sudden fall back into the electron’s normal, more stable orbit that results in the glows that you see. As the electron falls into its usual orbit (or electron shell, see: http://en.wikipedia.org/wiki/Electron_shell) it gives off the remaining energy it had originally absorbed from the UV light, in the form of lower energy/higher wavelength, visible light.
So there you go, a nice and not too technical explanation on how UV causes fluorescence! As always, be sure to pass by our frequently updated blog for the latest and greatest UV information, and indeed the most up-to-date urine eradication systems and technologies!





Will a wavelength of 300nm-10nm make a phosphorescent material (glow powder) glow brighter and longer when light is removed?
Hi Colin,
Many thanks for your message. We’ve not tested wavelengths of 300-310nm (I presume you meant this light range) on our paint yet, so we can’t say whether or not it’s more effective for creating bright glows in the dark than typical 375-400nm UV lights! I have a feeling those lower nanometer wavelengths could be more effective, as they are higher energy, but whether the electrons of your particular glow powder material would be excited in the right way remains to be seen.
If you’re able to test it we’d love to know how you got on 😀
Best regards
Steve Grzywacz
[Co-Founder – PandoraStocks LTD]